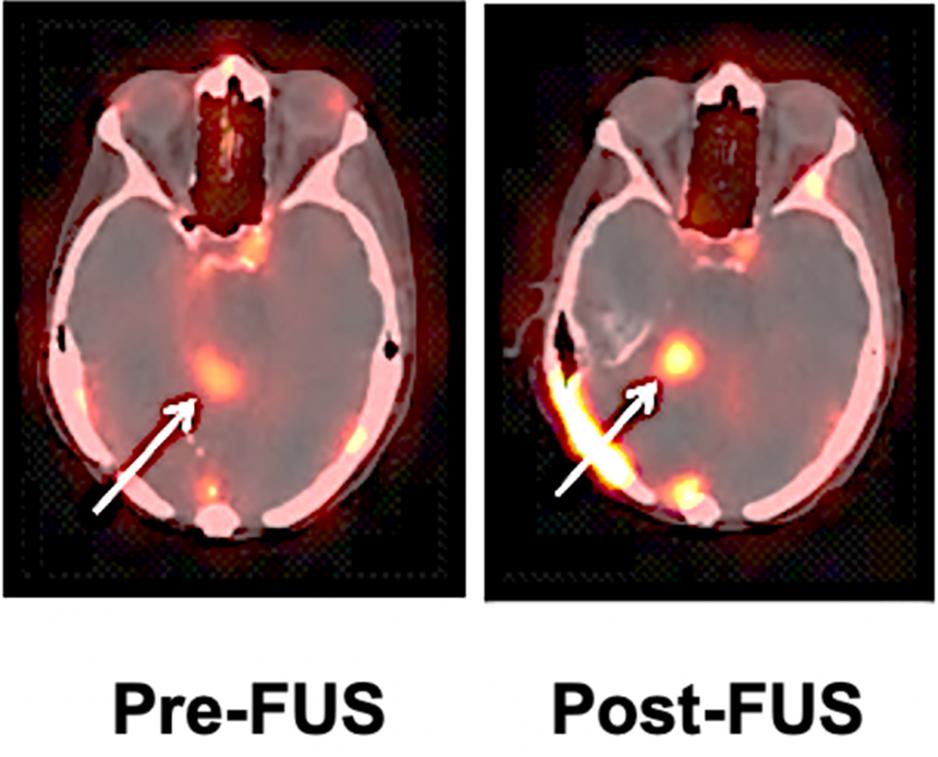
Reilly Lab’s radiopharmaceutical is helping an interdisciplinary research team visualize the uptake of Herceptin in brain metastases from breast cancer
A radiopharmaceutical drug developed at the Leslie Dan Faculty of Pharmacy (LDFP) is a key tool in a first-of-its-kind clinical trial at Sunnybrook Health Sciences Centre. Professor Raymond Reilly and his team linked a radioactive isotope to Herceptin, a drug used to treat HER2-positive breast cancer, to help a team of clinical researchers determine whether the drug can enter the brain and target brain metastases.
“This radiopharmaceutical drug developed at the Faculty of Pharmacy enables the research group to determine if a new approach to treating breast cancer metastasis in the brain is effective,” says Reilly, an expert in nuclear pharmacy. “It’s a really exciting and innovative trial.”
Herceptin successfully treats breast cancer tumours that display the HER2 protein, which are known as HER2-positive breast cancer, and has been critical in improving survival for this form of breast cancer. But longer survival means that more people with HER2-positive breast cancer are being diagnosed with brain metastases.
Metastatic tumours still display the HER2 protein, so there is potential that Herceptin could be effective for these tumours, except for one major problem: it can’t easily cross the blood-brain barrier, a protective physiological barrier that prevents drugs and other substances from entering the brain.
Reilly is collaborating with Dr. Nir Lipsman, a neurosurgeon, scientist, and Director of the Harquail Centre for Neuromodulation at Sunnybrook, and Dr. Kullervo Hynynen, a medical physicist and Vice President of Research and Innovation at Sunnybrook. They’re leading a clinical trial to examine whether ultrasound precisely focused on the region of the brain where the tumour is located can disrupt the blood-brain barrier to allow Herceptin to enter the brain and reach the tumour.
They are using magnetic resonance imaging (MRI) to determine whether focused ultrasound disrupted the blood-barrier barrier, but they needed Reilly and his team to help answer a central question of the study.
“The big challenge faced by the clinical research team was how to determine whether Herceptin can actually reach the brain metastasis at an increased level once they apply the focused ultrasound,” Reilly says.
Radiolabelled drug can be tracked and imaged in the brain
Reilly’s team attached a radioactive isotope to Herceptin so that the drug can then be imaged by single photon emission computerized tomography (SPECT), a common imaging technique used in nuclear medicine.
In the clinical trial, the patient first receives a dose of radiolabelled Herceptin without focused ultrasound to evaluate the baseline uptake of Herceptin into the tumour, then a couple of weeks later receives radiolabelled Herceptin and focused ultrasound. After each administration of the radiopharmaceutical, the patient has a SPECT scan, which allows the team to determine whether the radiolabelled Herceptin has entered the brain and been taken up by the tumour. The hope is that the focused ultrasound will improve the uptake of Herceptin into the tumour in the brain compared to the baseline study and, thus, demonstrate that focused ultrasound is an effective approach to help Herceptin enter the brain.
The team has completed the intervention for three patients and plans to test about 10 altogether. So far, Reilly says that the images reveal that focused ultrasound has increased the uptake of Herceptin into the tumour in the brain, but additional patient studies are required to confirm these promising findings.
“With the radiopharmaceutical developed at U of T, our collaboration with the clinical team at Sunnybrook is helping to answer a central question,” Reilly adds. “Researchers have already demonstrated that focused ultrasound disrupts the blood-brain barrier, but this study will determine whether it improves the uptake of Herceptin into the tumour in the brain.”
Once the team has a better understanding of whether focused ultrasound is effective in getting Herceptin into the brain, Reilly plans to study whether it could also be an effective treatment for HER2-positive brain metastases. The radioactive isotope linked to Herceptin could treat the tumour with radiation in addition to the effects of the Herceptin drug itself on the tumour.
Reilly, a former practising nuclear pharmacist with a strong interest in patient care, aims to support more clinical trials of radiopharmaceuticals in the future. In fact, a few years ago he received a $1.3 million grant from the Canadian Foundation for Innovation to build a Good Manufacturing Practices (GMP) facility, scheduled to open in late February, at the LDFP to facilitate creating radiopharmaceuticals for clinical trials. He also leads the Faculty’s Centre for Pharmaceutical Oncology, which helps to foster collaborations within the university and clinical oncology community to advance cancer research.
“Cancer research is very interdisciplinary, and this clinical trial is a classic example,” says Reilly. “It is led by a neurosurgeon, who is collaborating with a medical physicist, and they needed a pharmaceutical scientist like myself for it to come together. This collaboration is very emblematic of cancer research.”
The development of the radiopharmaceutical at the LDFP was supported by a grant from the Canadian Cancer Society. The clinical trial is funded by the Focused Ultrasound Foundation and the Harquail Centre for Neuromodulation at Sunnybrook.
By Eileen Hoftyzer
More News
Image

Welcoming Ivy Lam as Academic Lead in Climate, Health & Sustainable Care
Assistant Professor Lam will guide the Leslie Dan Faculty of Pharmacy's efforts to embed environmental sustainability across the Faculty.
Read More
Image

Pharmacy alum’s research shows how full-scope practice improves cancer care
Honoured with a national award, Adrian de Boer says his residency experience was a powerful reminder that he's making a meaningful change to the pharmacy profession.
Read More
Image

Pharmacy alum passionate about helping community pharmacists practice to full scope
As a pharmacy leader at Rexall, Heidi Wittke uses frontline experience to lead initiatives that improve patient care
Read More