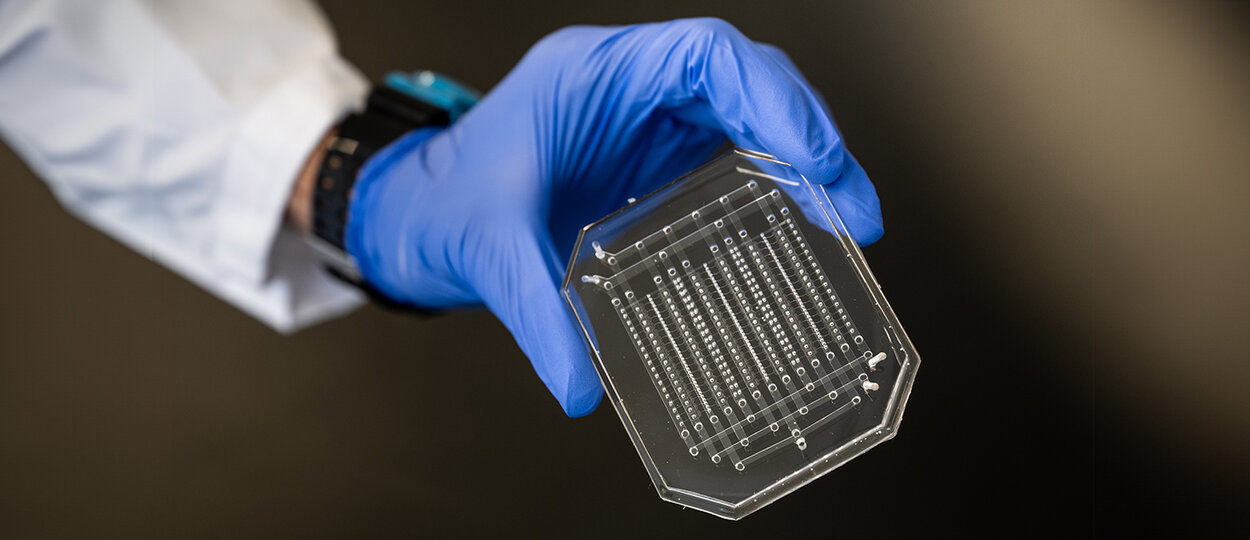
Microfluidic droplet generation device fabricated by PhD student Sushant Singh in CRAFT Device Foundry. This device is used to make bioinks for 3D printing human skin sheets. (photo credit: Dahlia Katz).
Nanomedicine is having a moment. From being used to deliver mRNA vaccines for COVID-19 and delivering targeted therapies for cancer, it seems like potential applications of nanomedicine are limitless.
“Nanomedicine has really made big strides in recent years. It has proven to have great potential and it can lead to therapies that can make a big difference in the world, like the COVID-19 vaccine and many others,” says Hagar Labouta, assistant professor (status) at U of T’s Leslie Dan Faculty of Pharmacy and Institute of Biomedical Engineering. “But nanomedicine still has more potential, and there are different areas that we haven’t fully explored.”
To explore and fully realize the potential of nanomedicine, scientists need research tools that help them study nanoparticles in a more accurate model. In response, the field of microfluidics – in which tiny amounts of fluid are studied in specialized devices – is growing and allowing researchers to tackle new challenges in fields including medicine, engineering, and pharmacy.
In pharmaceutical research, microfluidic technologies allow researchers to transform drug synthesis, screening, and delivery into a format that allows samples to be processed and tested continuously,” says Axel Guenther, professor in U of T’s department of mechanical and industrial engineering and co-director of the Centre for Research and Applications in Fluidic Technologies (CRAFT)
This transition can integrate computational drug design and machine learning, and promises to reduce the time and cost needed to identify promising new drug candidates and ultimately enable more flexible manufacturing of biopharmaceuticals.”
CRAFT is a research partnership between U of T and the National Research Council of Canada that aims to advance the development, commercialization and adoption of microfluidic medical devices. CRAFT’s scientists, technical staff and nationally unique research facilities are located at U of T and at the NRC’s campus in Boucherville, Quebec. Researchers with expertise in bioengineering, chemistry, and pharmaceutical sciences, collaborate across fields -- a critical approach to moving the new technologies forward.
“Regulators are now considering microfluidic technologies, particularly organ-on-a-chip systems, for routine use in drug development. The deep expertise of the scientists at the Leslie Dan Faculty of Pharmacy will be key for our efforts to have impact.”
“Developing powerful new microfluidic technologies requires a highly collaborative and multidisciplinary effort. It requires engineers and physical scientists who are experts in microfluidic device design and manufacture often using industrial processes that are available at CRAFT’s open research facilities at U of T,” says Guenther. “Regulators are now considering microfluidic technologies, particularly organ-on-a-chip systems, for routine use in drug development. The deep expertise of the scientists at the Leslie Dan Faculty of Pharmacy will be key for our efforts to have impact.”
Microfluidic model helps researchers understand placenta
Hagar Labouta works at the intersection of nanomedicine and microfluidics. Her team uses organ-on-a-chip technology – 3D organ tissue models in a small microfluidic device that mimic human physiology – to understand how nanoparticles interact with their environments in a realistic model. The goal is to design new therapies to help underserved populations, specifically pregnant people.
“We work at the intersection between nanomedicine, microfluidics, and molecular biology in order to understand the behaviour of the nanoparticles when they interact with biological barriers like the placenta, in order to design safe and effective therapies for pregnant women and fetuses,” says Labouta, who is also a scientist at Unity Health’s Keenan Research Center for Biomedical Science. “The placenta is a poorly understood biological barrier, especially when it comes to drug delivery. To make it even more complicated, the placenta changes throughout pregnancy, so it’s very difficult to find experimental models of the placenta.”
Labouta began her research career studying nanoparticles for drug delivery. She was a post-doctoral fellow at the University of Calgary when she began to realize the importance of fluid dynamics in nanomedicine. Understanding how fluids travel and interact with their environments ultimately impacts how successfully drugs can be delivered to their target tissue.
Her current research program, based at Unity Health Toronto, is developing a “placenta-on-a-chip” model to bypass the need for animal models, but still have the ability to study how nanoparticles interact with the placenta in a realistic model.
In collaboration with researchers at CRAFT, her team is now working to make the model more complex and realistic. The goal is that a model of the placenta, with all its complexities, can then be used to study how nanotherapies interact with the placenta, thereby better informing drug development and formulation research.
“For nanomedicine to reach its full potential, we need to have a better understanding of how nanoparticles behave in inherent biological environments, not just when they are separated from the biological environment.”
“For nanomedicine to reach its full potential, we need to have a better understanding of how nanoparticles behave in inherent biological environments, not just when they are separated from the biological environment,” says Labouta. “I’m trying to develop that environment on a small lab-based model that we can use to understand how nanoparticles interact with the barrier, in presence of other components of relevance in the body.”
Artificial intelligence and microfluidic technology rapidly accelerate protein discovery
Yufeng Zhao, assistant professor (status) at the Leslie Dan Faculty of Pharmacy and an NRC research officer based at CRAFT at U of T, is using his expertise in microfluidics to tackle a different problem: how to identify new proteins tailored to specific uses, including therapeutics and diagnostics.
As the building blocks of life, proteins can be used for many purposes in health care – if the right one can be found. But using traditional methods of identifying and testing large libraries of proteins for their effectiveness for a given purpose is time consuming and expensive.
Zhao, in collaboration with Keith Pardee, associate professor at the Leslie Dan Faculty of Pharmacy, and other researchers at CRAFT, is designing new tools that use microfluidics, artificial intelligence (AI) and robotics to screen large protein libraries to examine the therapeutic effectiveness of a protein for a specific condition and iteratively select new proteins that have the desired effects. He is also using microfluidics to develop fluorescent biosensors, which are proteins that light up in the presence of specific molecules and can be used in diagnostics, drug screening and studying disease mechanisms.
“Traditional screening methods relying on manual pipetting and multi-well plate testing can screen a library of 1,000 to 10,000 variants per day, which is time-consuming and labor-intensive. Microfluidic technologies are much higher throughput, allowing us to screen 1 to 10 million variants in a day,” says Zhao. “For my research, microfluidics and AI methods are very efficient, accelerating the development of protein therapeutics for treatment of diseases and biosensors for diagnostics and drug screening.”
AI can help microfluidic technology become even more efficient. In a related project, Zhao is developing AI that can predict a protein’s function based on its genetic sequence, which will help to “pre-screen” proteins to identify those most likely to have the desired effect before screening in a microfluidic tool, further improving the efficiency.
As with Labouta, Zhao hopes that his work to develop improved research tools will ultimately lead to better patient care.
“I hope my research can lead to much more effective pipelines for the discovery of proteins that can be used by clinician-researchers and others to design proteins according to their needs for broader pre-clinical and clinical applications,” says Zhao.
More News
Image

PharmSci student’s research advances medication safety during pregnancy
Diana Nelles was inspired by women in pharmacology research to advance knowledge in understudied populations.
Read More
Image

LIGAND-AI aims to transform early drug discovery through machine learning
Assistant Professor Rachel Harding is contributing to global initiatives to generate data needed to advance and accelerate drug discovery.
Read More
Image

Exploring pharmacy: A summer camp experience for high school students
Now in its second year, Pharmacy Summer Camp continues to give high school students hands-on experiences in pharmacy and pharmaceutical sciences.
Read More