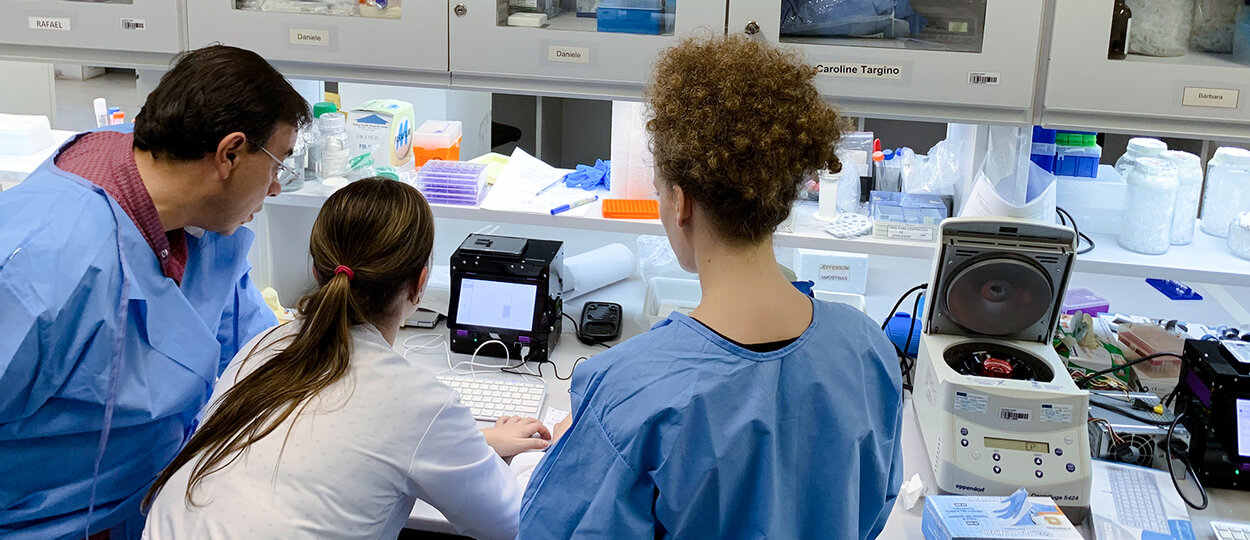
An international team of researchers, headed by experts from the University of Toronto’s Leslie Dan Faculty of Pharmacy, has led one of the first field trials for a synthetic biology-based diagnostic using patient samples. This work, conducted on-site in Latin America, reveals the potential for cell-free synthetic biology tools and companion hardware for providing rapid, de-centralized, and low-cost patient testing for infectious diseases like the Zika virus.
Study results, published in Nature Biomedical Engineering, show that the novel diagnostic platform has analytical specificity and sensitivity equivalent to a US Centre for Disease Control PCR test for Zika and a diagnostic accuracy of 98.5 per cent with 268 patient samples collected in Recife,
“We see emerging diagnostics, like the paper-based tests we’ve developed, as having tremendous near-term potential to augment existing PCR capacity, improve equity in access to health care, and aid in the responses to public health crises,” said Keith Pardee, associate professor in the department of pharmaceutical sciences, Leslie Dan Faculty of Pharmacy, University of Toronto.
Portable diagnostics aim to improve future outbreak response
Prior to the current global COVID-19 pandemic, the 2015/2016 outbreak of Zika virus in Latin America emphasized the urgent need for rapid and low-cost testing that can be deployed beyond the reach of centralized diagnostic labs, explains Pardee who has a Canada Research Chair in Synthetic Biology and Human Health. “We were investigating and developing this technology well before the COVID-19 pandemic brought these issues to light at the global level. We’ve now been able to apply it and validate it in a region of endemic disease, which is really promising because these tools are meant to enable health systems to better respond to future
Video file
Researchers from the international team led by experts from U of T's Leslie Dan Faculty of Pharmacy test the portable diagnostic technology onsite in Recife, Brazil
Dr. Lindomar Pena, department of virology, Oswaldo Cruz Foundation (Fiocruz), led the Brazilian team that collaborated on the project. "This robust diagnostic platform displayed desirable features to be used in developing countries such as Brazil and in laboratories with basic infrastructure. We hope it can be further developed and deployed in the Brazilian network of public health laboratories to diagnose Zika patients, trace contacts and identify hot-spot areas with active community transmission,” he said.
The portable diagnostic platform is a combination of a cell-free, paper-based test and a field-ready companion device that allows data to be collected using
U of T Entrepreneurship community supports health start-ups
Guo, Çiçek and Karlikow successfully moved their work with the Pardee lab to establish health tech startups, LSK Technologies and En Carta Diagnostics, respectively. The entrepreneurship and incubator community at U of T was instrumental in this process.
In particular Guo and Çiçek, collaborated closely with H2i and UTEST. “Both H2i and UTEST were great in terms of making introductions and helping to expand our network,” said Çiçek, explaining that H2i helped the team figure out the road map from the regulatory and product design standpoint and UTEST helped with customer discovery so they could better understand and mitigate customer pain points.
“The entrepreneurship community at U of T helps foster regular and valuable discussions between entrepreneurs around their challenges,” said Karlikow who is now running En Carta Diagnostics from Paris, France. “With so much support available, it helps you to make the decision to start your company to push your vision and product forward,” she said.
Tackling the next challenges
Showing that the platform could be transported and accurately detect Zika virus in patient samples is a significant step forward in creating more accessible and de-centralized testing, says Pardee. However, the extraction of RNA from patient samples still requires liquid handling by skilled technicians at this stage. “With performance on patient samples now validated, we are tackling these next challenges, like sample preparation, so that the platform and PCR-like diagnostic capacity can be distributed more broadly into the communities where they are needed.”
“This was more than just the science involved, we made it applicable. We took it to the real-world, and it worked.”
The momentum from achieving this successful field-based patient trial will continue to move the team forward. “This step of translating the technology from the lab and applying it in a real-world setting was so important,” said Karlikow. “This was more than just the science involved, we made it applicable. We took it to the real-world, and it worked.”
More News
Image

Pharmacy Summer Camp gives high school students insight into pharmacy profession
A new summer camp based at the faculty will give high school students a range of experiences in pharmacy and pharmaceutical sciences.
Read More
Image

Team GloveLift wins 2025 Business Plan Competition with innovative medical device
PharmD students win $5,000 prize for their innovative medical device concept aimed at improving patient care.
Read More
Image

Faces of PharmSci: Kinda Karra
MScPhm student Kinda Karra, working with Clinician Scientist Carlo DeAngelis, is studying why some breast cancer patients experience pain after chemotherapy and how a small blood sample could help detect signs of this reaction.
Read More